FDA Approves New Cancer Drug for Clinical Trials Based on Nonanimal Data Only
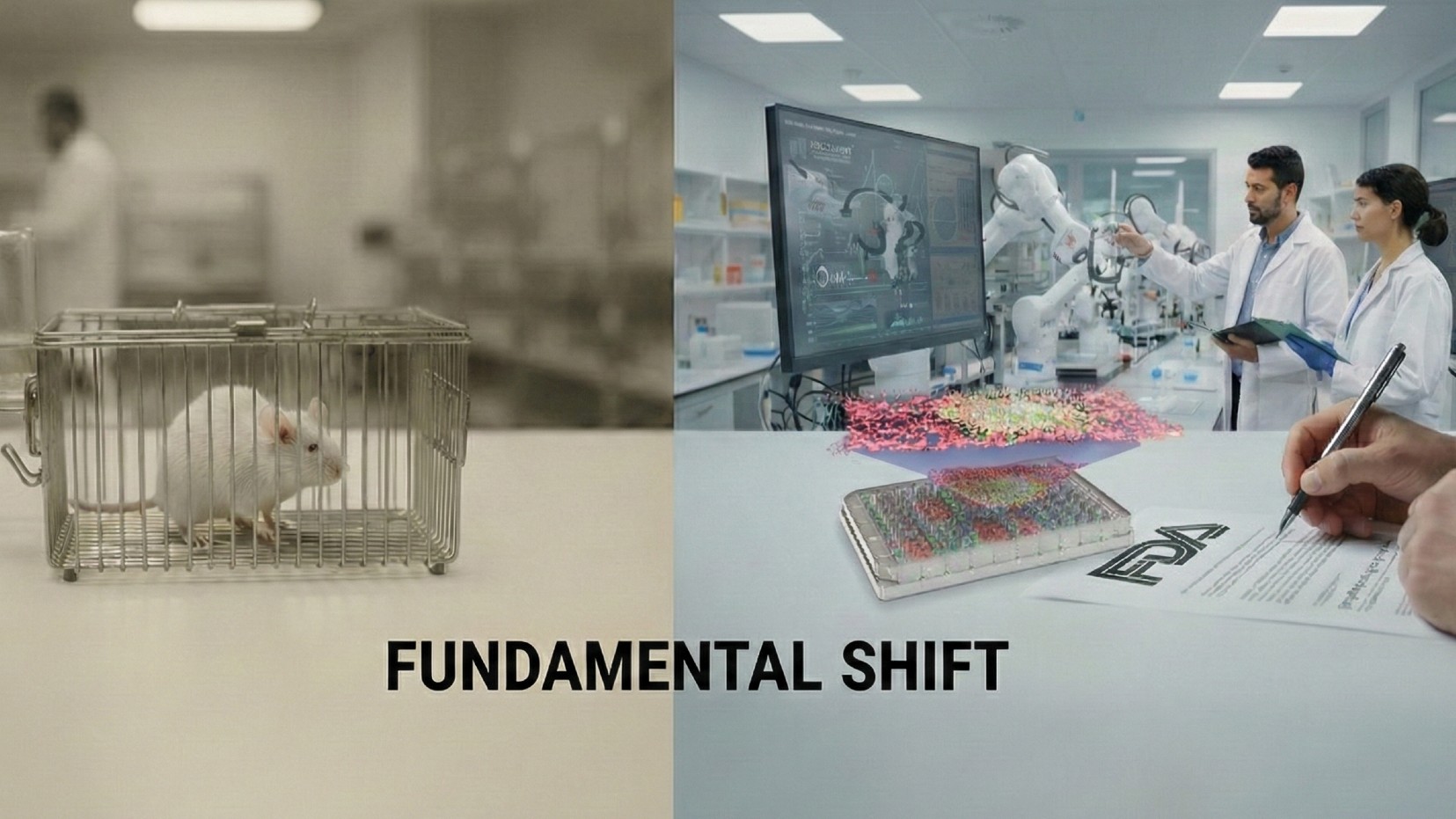
Study in a Sentence: The U.S. Food and Drug Administration approved an Investigational New Drug (IND) application using efficacy data solely from Qureator’s human vascularized organoid model, moving an oncology drug to human clinical trials without any animal tests for the first time.
Healthy for Humans: Due to species-specific biological differences, approximately 95 percent of cancer drugs found safe and effective in animal tests fail when they reach human clinical trials. Using innovative, human-based methods, such as organoids and organ chips, can overcome this poor preclinical predictiveness and improve drug attrition rates.
Redefining Research: The FDA’s approval of an IND application using only human-based methods is a significant landmark in drug development—ushering in a new regulatory precedent in preclinical testing without the use of animals. Qureator’s 3D vascularized tumor organoid model accurately recreates human vascular structures and immune environments. During the preclinical stage, this technology is enhanced by the Quricore AI platform that integrates human data, improving clinical predictability over animal tests and existing organoid models.