Statement by the Physicians Committee | New Paper Sets a Roadmap to End Three Lethal Animal Tests
Statement by Kristie Sullivan, M.P.H., Physicians Committee Vice President of Toxicology
Acute toxicity refers to potentially lethal toxicity caused by a single dose of a chemical or drug. Currently, tens of thousands of animals are killed each year in acute systemic tests—called Lethal Dose 50 tests—by being forced to inhale or consume chemicals or drugs, or by having the chemicals spread on their saved skin for several hours. Animals used in these tests, sadly, include dogs, cats, rats, mice, and rabbits.
Even more frustrating is that these tests don’t often tell us how humans might react to these chemicals. There are clear differences between species in the amounts, or dose levels, at which many chemicals or drugs are toxic. However, the tests are required to be conducted by several government agencies in the United States and around the world.
To identify the steps that need to be taken to replace these tests as quickly as possible, the Physicians Committee co-sponsored a workshop at the National Institutes of Health in September 2015 called “Alternative Approaches for Identifying Acute Systemic Toxicity: Moving from Research to Regulatory Testing.” The paper summarizing the discussions and recommendations from the workshop has just been published.
The workshop brought together more than 60 toxicologists from the Department of Defense, the Environmental Protection Agency, the Department of Transportation, the Consumer Product Safety Commission, and several chemical companies and universities. These experts presented the laws and regulations which currently require these tests, scientific methods to replace the tests, and ideas for changing those laws and regulations.
The report contains important information relevant to acute toxicity testing, including example testing approaches considered by federal agencies, organizations which use LD50 data to set exposure limits and other reference values, and information about other similar efforts underway in other regions, such as the European Union.
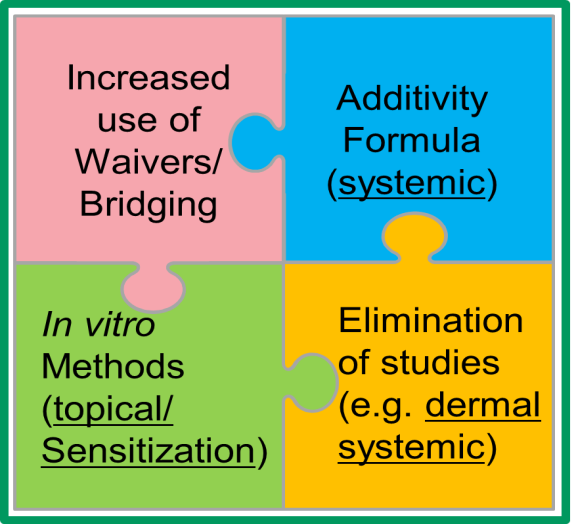
One recommendation of the workshop, which was that dermal LD50 studies on new pesticide formulations need not be conducted, has already been implemented. In November, the EPA announced the finalization of a rule that allows the waiving of dermal LD50 studies for pesticide formulations, saving 2,500 rabbits each year.
The report makes several additional recommendations, including outlining Adverse Outcome Pathways (AOPs) to understand how severely toxic chemicals harm humans, building publicly available databases to take advantage of what we already know, and reaching out to international regulatory agencies to ensure any progress we make here in the United States is matched abroad. Progress is being made on these and other recommendations, and the workshop sponsors will hold a check-in meeting to review ongoing progress at the Society of Toxicology meeting in March 2017.
Founded in 1985, the Physicians Committee for Responsible Medicine is a nonprofit organization that promotes preventive medicine, conducts clinical research, and encourages higher standards for ethics and effectiveness in education and research.