Physicians Group Recommends Updates to 235 FDA Regulations to Advance Innovative Human-Relevant Science
Image Credit: FDA.
A growing number of scientists are realizing that biological and physiological differences between—and even among—species are leading to failures in the development of safe and effective medicines.
Scientists from a variety of backgrounds are working to develop better methods to evaluate safety. And they have been successful in elevating our abilities to investigate how investigational new medicines interact with human cells, tissues, and biological and physiological processes.
As is often the case, law and policy did not keep pace with science. Now, we are at a standstill, where many of these so called “human-based” scientific approaches already exist for evaluating medicinal safety, such as organ chips and computational methods, but they have not been fully utilized for multiple reasons.
One main hindrance is Food and Drug Administration (FDA) regulations.
Before the regulated industry can be expected to implement a new approach to answering a regulatory question for the FDA, industry must be confident that the FDA will accept the new approach.
One step towards implementation is regulators clearly communicating that human-based approaches, such as advanced in vitro and in silico approaches, are accepted if sufficiently evaluated. FDA may start this communication by broadening the regulations that currently require animal data.
FDA staff have stated informally at meetings, that the agency retains discretion to accept modern approaches to assessing safety that do not use animals. Certainly, the law of the land allows it.
The Federal Food, Drug, and Cosmetic Act (FFDCA) is the United States law that gives FDA the authority to regulate drugs. The FFDCA does not mandate animal data for pharmaceutical development. Rather, this mandate arises from FDA promulgated regulations.
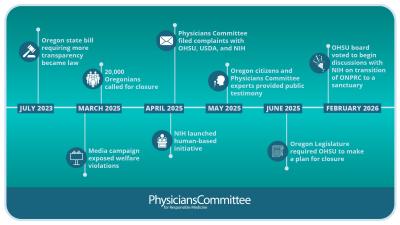
In September 2017, an FDA blog announced that a Regulatory Reform Task Force would review agency regulations and requested input on regulations that need to be modified or repealed because they place an burden on the regulated community. This was the opportunity we needed, as we had already been recommending that FDA broaden regulations that currently require animal data to also allow for the use of modern approaches that are based in human biology and physiology.
We combed through the Code of Federal Regulations and ultimately identified 235 regulations that should be changed because they place a burden on industry to use animals, even if a more predictive or cost-effective approach is available.
Our suggestions should be adopted because they remove this burden, help ensure the longevity of the regulation in the face of rapidly advancing science, and will help FDA improve product safety and meet its goal of replacing and reducing animal testing.
Making the proposed regulation changes would be consistent with FDA’s own participation in the National Institutes of Health (NIH) and Defense Advanced Research Projects Agency (DARPA) tissue chip collaborations and the vision for advancing the methods available to drug sponsors through FDA’s own Predictive Toxicology Roadmap.
Bookmark our Good Science Digest to stay updated on how the Regulatory Reform Task Force and FDA responds to our input.