FDA Unveils Predictive Toxicology Roadmap to Advance Human-Based Tests
Image Credit: FDA.
The Food and Drug Administration (FDA) has joined other drug development stakeholders in pushing for the development and implementation of more predictive preclinical testing approaches. Last week FDA unveiled its Predictive Toxicology Roadmap, which aims to transform the development, qualification, and integration of new toxicology methods across FDA centers.
Toxicology testing is crucial for the development of safe medical products that are regulated by FDA because it informs potential risk to humans. Traditionally, toxicity testing has used animals to make predictions about human outcomes. However, the quality of information gained from animal tests has been the subject of much debate.
For far too long animal-based toxicity tests have been accepted as the benchmark for testing despite a general failure to provide information that is sufficiently relevant to humans. For example, the FDA and drug development stakeholders have known for more than 13 years that approximately 92 percent of new medicines fail in humans after passing animal tests.
This Roadmap acknowledges the scientific advances made and establishes a plan for support and implementation.
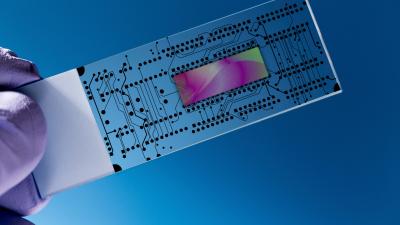
In an article released on the subject, FDA’s acting chief scientist stated, “Today, novel methods such as organs on a chip or mathematical modeling are being developed for toxicity testing that are generating unique opportunities to improve our ability to quickly and more accurately predict potential toxicities and reduce associated risks.”
The six-part Roadmap seeks to improve toxicity testing and formalizes FDA’s position on replacing/reducing animal testing.
The Roadmap establishes a Toxicology Working Group to facilitate communication among FDA centers. FDA knowledge will no longer be siloed in separate centers, but will rather be shared to help determine which approaches may be used for which purposes.
The group will identify gaps in current test methods and determine where newer approaches—such as organ chips and computer simulations—may be identified, further developed, scientifically evaluated, and implemented.
The group will also develop a Toxicology Seminar Series to facilitate agency-wide training on new testing approaches. We’ve pushed for this much-needed initiative through consistent input to FDA and ICCVAM, as agency scientists must build their knowledge-base, confidence and familiarity with modern approaches to toxicity testing.
The Roadmap encourages frequent and often communication with FDA regarding interest in using new methods. FDA should take this opportunity to update its regulations that mandate animal data, to ensure the Roadmap is properly implemented. Companies may not be willing to rely on the Roadmap in the face of inconsistent regulations.
The Roadmap solidifies FDA’s commitment to working with the stakeholder community, which has seemed to increase over the past year. In January 2017, FDA participated in a Preclinical Innovation and Patient Safety (PIPS) roundtable hosted by the Physicians Committee. The roundtable aimed to increase stakeholder collaboration to advance modern, predictive approaches. FDA has also collaborated with the National Center for Advancing Translational Sciences (NCATS) and the Microphysiological Systems Working Group of the IQ Consortium to provide input on tissue chip technology.
The Toxicology Working Group will track progress and provide annual reports to the chief scientist to increase oversight and accountability.
We applaud FDA for its transparency and vision set forth in the Roadmap, and look forward to continued collaboration to save human and animal lives.
Learn more from our The Hill op-ed and The Exam Room.